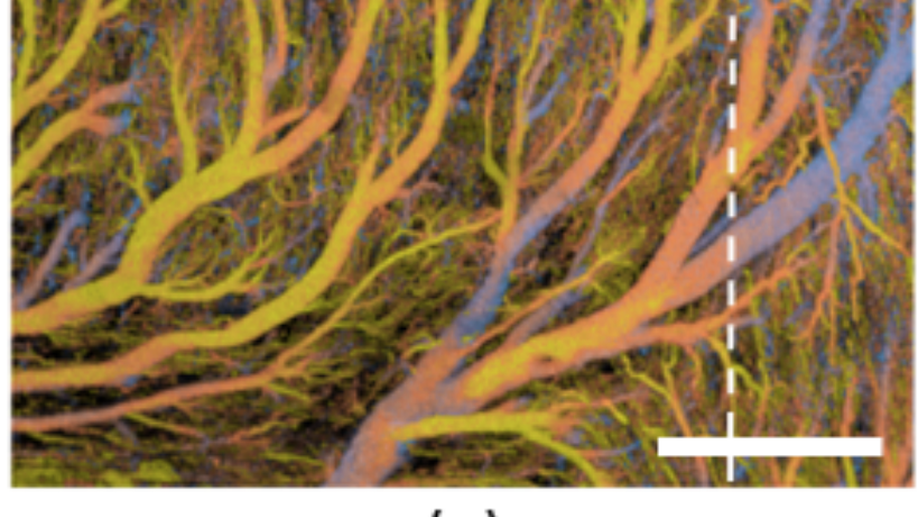
Quantifying Microvascular Structure in Healthy and Infarcted Rat Hearts Using Optical Coherence Tomography Angiography
Myocardial infarction (MI) is a life-threatening medical emergency resulting in coronary microvascular dysregulation and heart muscle damage. One of the primary characteristics of MI is capillary loss, which plays a significant role in the progression of this cardiovascular condition. In this study, we utilized optical coherence tomography angiography (OCTA) to image coronary microcirculation in fixed rat hearts, aiming to analyze coronary microvascular impairment post-infarction. Various angiographic metrics are presented to quantify vascular features, including the vessel area density, vessel complexity index, vessel tortuosity index, and flow impairment. Pathological differences identified from OCTA analysis are corroborated with histological analysis. The quantitative assessments reveal a significant decrease in microvascular density in the capillary-sized vessels and an enlargement for the arteriole/venule-sized vessels. Further, microvascular tortuosity and complexity exhibit an increase after myocardial infarction. The results underscore the feasibility of using OCTA to offer qualitative microvascular details and quantitative metrics, providing insights into coronary vascular network remodeling during disease progression and response to therapy.

Stacking thick perfusable human microvascular grafts enables dense vascularity and rapid integration into infarcted rat hearts
Fabrication of large-scale engineered tissues requires extensive vascularization to support tissue survival and function. Here, we report a modular fabrication approach, by stacking of patterned collagen membranes, to generate thick (2 mm and beyond), large, three-dimensional, perfusable networks of endothelialized vasculature. In vitro, these perfusable vascular networks exhibit remodeling and evenly distributed perfusion among layers, while maintaining their patterned, open-lumen architecture. Compared to non-perfusable, self-assembled vasculature, constructs with perfusable vasculature demonstrated increased gene expression indicative of vascular development and angiogenesis. Upon implantation onto infarcted rat hearts, perfusable vascular networks attain greater host vascular integration than self-assembled controls, indicated by 2.5-fold greater perfused vascular density measured by histological analysis and 5-fold greater perfusion rate measured by optical microangiography. Together, the success of fabricating thick, perfusable tissues with dense vascularity and rapid anastomoses represents an important step forward for vascular bioengineering, and paves the way towards more complex, large scale, highly metabolic engineered tissues.

Polarization sensitive optical coherence tomography with single input for imaging depth-resolved collagen organizations
Collagen organization plays an important role in maintaining structural integrity and determining tissue function. Polarization-sensitive optical coherence tomography (PSOCT) is a promising noninvasive three-dimensional imaging tool for mapping collagen organization in vivo. While PSOCT systems with multiple polarization inputs have demonstrated the ability to visualize depth-resolved collagen organization, systems, which use a single input polarization state have not yet demonstrated sufficient reconstruction quality. Herein we describe a PSOCT based polarization state transmission model that reveals the depth-dependent polarization state evolution of light backscattered within a birefringent sample. Based on this model, we propose a polarization state tracing method that relies on a discrete differential geometric analysis of the evolution of the polarization state in depth along the Poincare sphere for depth-resolved birefringent imaging using only one single input polarization state. We demonstrate the ability of this method to visualize depth-resolved myocardial architecture in both healthy and infarcted rodent hearts (ex vivo) and collagen structures responsible for skin tension lines at various anatomical locations on the face of a healthy human volunteer (in vivo).
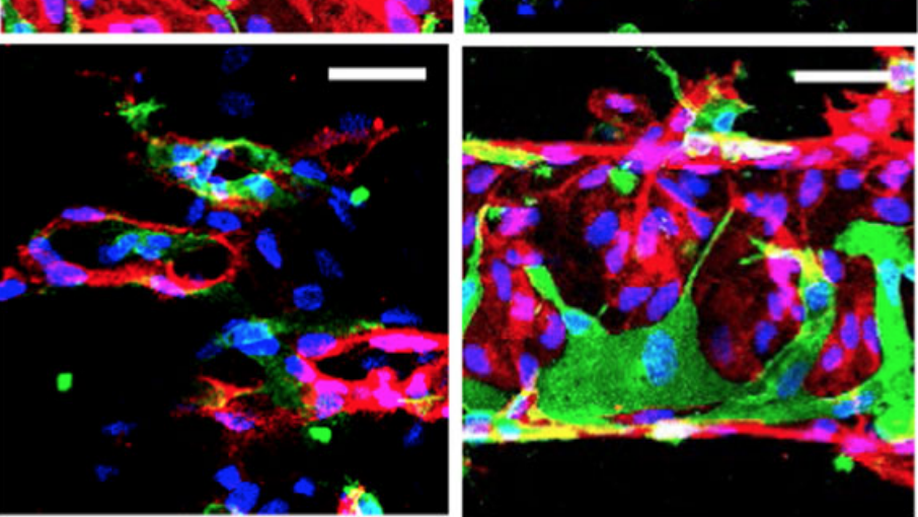
Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts
Vascularization and efficient perfusion are long-standing challenges in cardiac tissue engineering. Here we report engineered perfusable microvascular constructs, wherein human embryonic stem cell-derived endothelial cells (hESC-ECs) are seeded both into patterned microchannels and the surrounding collagen matrix. In vitro, the hESC-ECs lining the luminal walls readily sprout and anastomose with de novo-formed endothelial tubes in the matrix under flow. When implanted on infarcted rat hearts, the perfusable microvessel grafts integrate with coronary vasculature to a greater degree than non-perfusable self-assembled constructs at 5 days post-implantation. Optical microangiography imaging reveal that perfusable grafts have 6-fold greater vascular density, 2.5-fold higher vascular velocities and >20-fold higher volumetric perfusion rates. Implantation of perfusable grafts containing additional hESC-derived cardiomyocytes show higher cardiomyocyte and vascular density. Thus, pre-patterned vascular networks enhance vascular remodeling and accelerate coronary perfusion, potentially supporting cardiac tissues after implantation. These findings should facilitate the next generation of cardiac tissue engineering design.